The required substance is H2S.
H2S + 2FeCl3 = S + 2FeCl2 + 2HCl
H2S + 2AgNO3 = Ag2S![]() + 2HNO3
+ 2HNO3
The methyl radical - CH3 displays +I-effect and, hence, increases the activity of aromatic system in electrophilic substitution compared to that in benzene. The chlorine atom displays both –I and +M-effects; it reduces the activity of aromatic system, but this reduction is not as strong as in the case of nitro group - NO2, which displays only negative (–M) electronic effect.
So, the required list is: toluene > chlorobenzene > nitrobenzene.
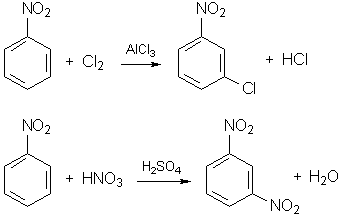
The reactions are (nitro group - NO2 directs the substitution into meta-position):
To solve the problem, we should express the ratio of rate constants at temperatures T2 and T1 using the Arrhenius equation. For the first reaction:
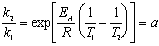 .
.
Activation energy of the second reaction is half of that of the first reaction: EA' = EA / 2; hence, the ratio for the second reaction is:
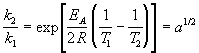 .
.
X - CH3, Y - COOH.
The reactions:
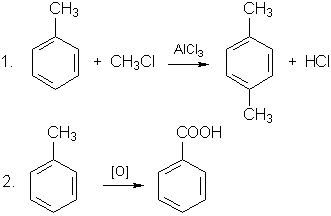
(5C6H5CH3 + 6KMnO4 + 9H2SO4 = 5C6H5COOH + 3K2SO4 + 6MnSO4 + 14H2O).
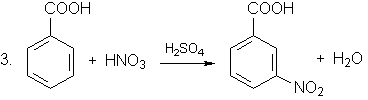
P2O3 + 2H2SO4(conc) + H2O = 2H3PO4 + 2SO2 (P+3 ![]() S+4)
S+4)
SO2 + Ba(OH)2 = BaSO3![]() + H2O (S+4
+ H2O (S+4 ![]() Ba+2)
Ba+2)
BaSO3 + 3C = BaS + 3CO (upon heating) (Ba+2 ![]() C+2).
C+2).
HCOOH + KOH = HCOOK + H2O
CH3COOH + KOH = CH3COOK + H2O
HCOOK + Ag2O ![]() 2Ag
2Ag![]()
H2CO + 2Ag2O ![]() 4Ag
4Ag![]()
Let ![]() (HCOOH) = x,
(HCOOH) = x, ![]() (CH3COOH)
= y,
(CH3COOH)
= y, ![]() (H2CO) = z. It follows from reactions with KOH that
(H2CO) = z. It follows from reactions with KOH that
![]() (KOH) = x + y = 18.7.
1.07.
0.084 / 56 = 0.03 mol.
(KOH) = x + y = 18.7.
1.07.
0.084 / 56 = 0.03 mol.
It follows from reactions with Ag2O that
![]() (Ag2O) = 2x + 4z = 9.72 / 108 = 0.09 mol.
(Ag2O) = 2x + 4z = 9.72 / 108 = 0.09 mol.
The mass of the initial solution is: 46x + 60y + 30z = 2.33 g.
Solving the system of three linear equations, we find: x = 0.005, y = 0.025, z = 0.02.
The molar fractions of the components are: 10% HCOOH, 50% CH3COOH, 40% H2CO.
![]() (Cu) = 4.8 / 64 = 0.075 mol;
(Cu) = 4.8 / 64 = 0.075 mol;
![]() (NaOH) = 100.
0.1 / 40 = 0.25 mol.
(NaOH) = 100.
0.1 / 40 = 0.25 mol.
| 0.075 | 0.15 | |||||||
| Cu | + | 4HNO3 | = | Cu(NO3)2 | + | 2NO2 | + | 2H2O |
| 0.15 | 0.075 | 0.075 | ||||||
| 2NO2 | + | 2NaOH | = | NaNO2 | + | NaNO3 | + | H2O (NaOH is an excess) |
The maximum amount of iodine can be obtained through complete reduction of nitrite and nitrate ions to NO:
| 0.075 | 0.0375 | |||||||||
| 2NO2– | + | + | 4H+ | = | I2 | + | 2NO | + | 2H2O |
| 0.075 | 0.1125 | |||||||||
| 2NO3– | + | + | 8H+ | = | 3I2 | + | 2NO | + | 4H2O |
![]() (I2) = 0.0375 + 0.1125 = 0.15 mol, m(I2) = 0.15.
254 = 38.1 g.
(I2) = 0.0375 + 0.1125 = 0.15 mol, m(I2) = 0.15.
254 = 38.1 g.
See also:
Chemistry Department of Moscow State University