Extended abstract for "18th Symposium on Halogenated Environmental Organic Pollutants Organohalogen Compounds - Dioxin'98", Stockholm, Sweden, August 17 - 21, 1998, in Organohalogen Compounds, 36, pp 197-200.
On Principles of Thermodynamic Modeling of Dioxins Formation and Behavior in Thermal Processes
Alexander K. Zaytsev, Leopold I. Leontiev, Yulian S. Yusfin,
Gleb V. Belov*, Vladimir S. Iorish*
Moscow Steel and Alloys Institute, Leninsky pr., 4, Moscow, Russia
* Glushko Thermocenter of RAS, IVTAN Association, Izhorskaya 13/19, Moscow 127412, Russia
Introduction
The investigation of formation and behavior in thermal processes of polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofurans (PCDF), both of which are often named "dioxins", is important in two aspects. On the one hand, various thermal technologies including the incineration of the domestic and industrial wastes are recognized as a significant source of the dioxins emission into environment. On the other hand, these technologies are recognized as one of the main methods of the annihilation of these extremely toxic compounds. So, the theoretical analysis and prediction of PCDD/F formation in the wide range of temperature values, redox conditions and the source composition is of primary importance. The best way of obtaining of this information is the thermodynamic modeling (TM) based on calculation of equilibrium composition [1]. The equilibrium concentrations of PCDD/F should be regarded as maximum available values, what is the goal of an ecological examination. It should be noted that TM allows take into account some kinetic restrictions by excluding some substances from the list of possible products or by assigning the fixed values for the concentrations of some products.
To apply the TM to the problem discussed one should create a database on thermodynamic properties of individual substances and develop the algorithms and related software. The database and software should make it possible to accomplish all necessary calculations, informativity and the simplicity of the analysis of the results of calculations, because the hundreds of substances may be regarded as possible products. The solution of these tasks led to the development of the new version of IVTANTHERMO for Windows, which is supplied with the extensive database on thermodynamic properties of substances. Now the database contains information about 2500 substances formed by 96 chemical elements [2].
Materials and Methods
The system examined in present study was C + 1.5H + 0.5Cl, what is the typical composition of PVC. The calculations were accomplished for the fixed pressure 0.1 MPa in the temperature range 573 K - 1773 K with the variation of O/C ratio from 0.1 to 2.1. The sum of the mole parts of all isomer groups of chlorinated dioxins (å
XDi) and furans (å
XFi) was taken as a main quantitative characteristic of PCDD/F concentration. For the qualitative characteristic of PCDD/F composition the mole parts of various isomer groups (XDi/å
XDi and XFi/å
XFi) were taken. Some important results are shown in Table 1 and Figures 1, 2.
Table 1
Results of calculations system C+1.5H+0.5Cl+0.5O for different variants of modeling.
|
COMPUTATION MODEL |
-
lg XO2 |
-
lg S
XF |
-
lg S
XD |
 *
*
|
|
N |
EXCLUDED SUBSTANCES |
773 K |
1273 K |
773 K |
1273 K |
773 K |
1273 K |
773 K |
1273 K |
|
1 |
-
|
27.62 |
19.35 |
42.34 |
34.63 |
53.55 |
44.66 |
11.21 |
10.03 |
|
2 |
C(gr) |
30.06 |
22.63 |
18.86 |
16.52 |
31.29 |
28.19 |
12.43 |
11.67 |
|
3 |
C(gr), CnHm (n³
22) |
30.48 |
22.94 |
16.20 |
14.84 |
28.85 |
26.67 |
12.65 |
11.83 |
|
4 |
C(gr), CnHm (n³
17) |
31.11 |
23.43 |
12.58 |
12.24 |
25.54 |
24.32 |
12.96 |
12.08 |
|
5 |
C(gr), CnOm |
19.16 |
10.51 |
12.89 |
10.63 |
19.73 |
16.20 |
6.84 |
5.57 |
|
6 |
C(gr), CnOm, CnHm (n³
22) |
19.48 |
10.55 |
8.55 |
7.58 |
15.53 |
13.15 |
6.98 |
5.57 |
|
7 |
C(gr), CnOm, CnHm (n³
17) |
19.37 |
10.53 |
6.32 |
5.41 |
13.17 |
10.95 |
6.85 |
5.54 |
|
8 |
variant (7) + 100 Ar |
20.09 |
11.50 |
9.29 |
8.52 |
16.59 |
14.60 |
7.30 |
6.08 |
*
 = lgS
XF -
lgS
XD
= lgS
XF -
lgS
XD
The dioxins and furans are thermodynamically unstable, as well as the other organic compounds, and in presence of graphite in the system modeled (Table 1, model 1) the calculated concentrations of PCDD/F are negligibly small. For the model 1 the only significant products are (C(gr), H2, HCl, CO, CO2. So, the TM of formation and behavior of organic substances including the PCDD/F is only possible when graphite is expelled from the list of the possible products. From the chemical point of view this means that the pyrolysis process is partially prohibited. Though the concentrations of PCDD/F in the absence of graphite are much higher (Table 1, model 2) the absolute values of the concentrations are still very small. Now, the main carbon containing products are the aromatic hydrocarbons CnHm where n > 30, which are the predecessors of the graphite formation due to pyrolysis. By means of the "kinetic restriction" on these stages of pyrolysis we successively expelled from the list of possible products CnHm substances with the maximum number of the carbon atoms (Table 1, models 3,4). This resulted in some growth of the PCDD/F concentrations but the unrealistic difference between the concentrations of furans and dioxins (more than 10 orders) still takes place. Besides, in models 1-4 the only significant PCDD/F are the monochloric isomers F1 and D1, while the concentrations of the other isomer groups are much lower. These results are in a bad agreement with the known experimental data.
The significant exceeding of calculated concentrations of furans over the concentrations of dioxins can be explained at closer examination of chemical equilibrium among the isomer groups and oxygen (Table 2). Taking into account the mass action law one can express the difference between the concentrations of the two isomer groups of furans and dioxins as
 = lg XFi -
lg XDi = -
lg KP -
0.5 lg XO2
= lg XFi -
lg XDi = -
lg KP -
0.5 lg XO2
|
|
|
|
|
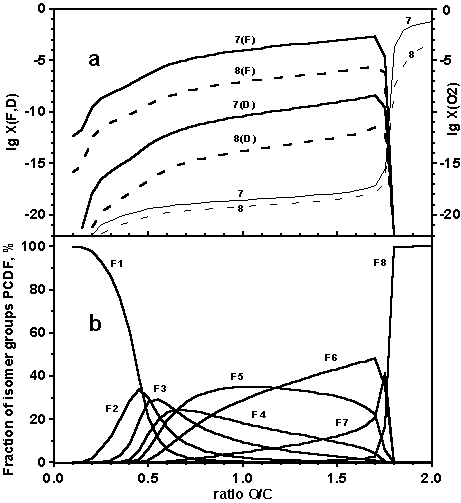
Fig.1 Variation of (a) sums of mole fractions for all isomer groups of PCDF (F), PCDD (D) and mole fraction of oxygen (computation models: 7 - solid, 8 - dot) and (b) fractions of different isomer groups of PCDF, % ( model 7) with amount of oxygen present for a fixed total pressure 1 atm and temperature 773 K. C:H:Cl ratio 1:1.5:0.5.
|
|
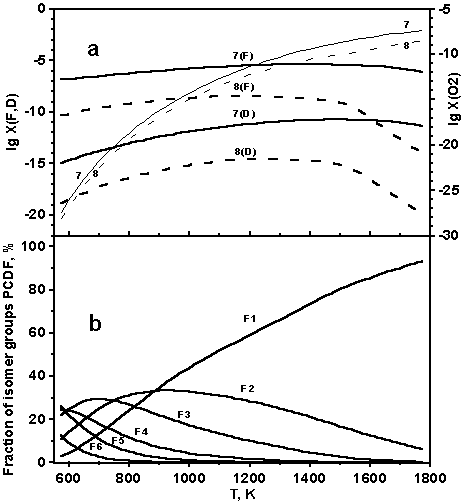
Fig.2 Variation of (a) sums of mole fractions for all isomer groups of PCDF (F), PCDD (D) and mole fraction of oxygen (computation models: 7 - solid, 8 - dot) and (b) fractions of different isomer groups of PCDF, % ( model 7) with temperature for a fixed total pressure 1 atm. C:H:Cl:O ratio 1:1.5:0.5:0.5.
|
The absolute values of lg Kp are relatively small (Table 2), so, the value of  is determined by lg XO2, which are approximately equal for the variants 1-4. Hence, the big difference between the equilibrium concentrations of PCDD/F can be explained by very low concentrations of O2.
is determined by lg XO2, which are approximately equal for the variants 1-4. Hence, the big difference between the equilibrium concentrations of PCDD/F can be explained by very low concentrations of O2.
Table 2
Values of lg Kp for the reactions Fx + 1/2O2 = Dx
|
T, K |
x = 1 |
x = 2 |
x = 3 |
x = 4 |
x = 5 |
x = 6 |
x = 7 |
x = 8 |
|
773 |
2.60 |
2.84 |
2.84 |
2.82 |
2.85 |
2.92 |
2.89 |
2.82 |
|
1273 |
-0.36 |
-0.14 |
-0.16 |
-0.16 |
-0.14 |
-0.09 |
-0.12 |
-0.16 |
|
1773 |
-1.60 |
-1.41 |
-1.44 |
-1.44 |
-1.42 |
-1.38 |
-1.40 |
-1.43 |
One may suppose that with the growth of O2 amount this difference will tend to diminish. However, even at O/C = 1 the sharp fall of PCDD/F concentrations occurs. This circumstance is caused by the complete oxidation of carbon to CO and CO2. It should be marked that the exclusion of furans from the list of products do not cause any change of concentrations of dioxins. Computed value of the oxygen concentration (and  ) may be changed by the exclusion of some of the main products that contain oxygen, and first of all CO and CO2. The last restriction may be explained by the fact that the oxidation process is not the equilibrium one and some small amount of C and O remains free in the system and may be redistributed among other products including PCDD/F.
) may be changed by the exclusion of some of the main products that contain oxygen, and first of all CO and CO2. The last restriction may be explained by the fact that the oxidation process is not the equilibrium one and some small amount of C and O remains free in the system and may be redistributed among other products including PCDD/F.
Results and Discussion
Results of calculations 5-7 in Table 1 obtained with the excluded carbon oxides show not only the significant growth of PCDD/F concentrations, but also a significant reduction of  . The chemical composition of the isomer groups becomes more complicated. Isomer groups F1 and D1 are not dominating and concentrations of other dioxins and furans are noticeable too (Figure 1b, 2b). Though the sum of concentrations of furans still significantly exceeds the sum of concentrations of dioxins the qualitative composition of these isomer groups is identical. In the deficit of oxygen the temperature growth does not influence significantly on PCDD/F concentrations, see Figure 2 where some growth of concentrations is noticeable. The results obtained show that PCDD/F are stable only in the reductive processes.
. The chemical composition of the isomer groups becomes more complicated. Isomer groups F1 and D1 are not dominating and concentrations of other dioxins and furans are noticeable too (Figure 1b, 2b). Though the sum of concentrations of furans still significantly exceeds the sum of concentrations of dioxins the qualitative composition of these isomer groups is identical. In the deficit of oxygen the temperature growth does not influence significantly on PCDD/F concentrations, see Figure 2 where some growth of concentrations is noticeable. The results obtained show that PCDD/F are stable only in the reductive processes.
And finally, we should mark that the proposed ways of TM do not pretend to be absolute and universal. Moreover, calculated valued of the equilibrium concentrations of PCDD/F should be treated from the qualitative point of view. The approach presented allows to determine some regularities of formation and behavior of these substances in thermal processes.
The work was supported by Russian Fund of Basic Researches, grant No 98-03-33021a.
References
- Thompson D.; Chemosphere. 1994, 29, 2583-2595.
- Gurvich, L.V., Veitz, I.V., et al. Thermodynamic Properties of Individual Substances. Fourth edition in 5 volumes, Hemisphere Pub Co. NY, L., vol. 1 in 2 parts, 1989, etc.
![]() *
*
