Proc. of the 5th Asian Thermophysical Properties Conference, Aug. 30 - Sep. 2, Seoul, Korea, pp. 583-586, 1998.
CRITICAL CONSTANTS ESTIMATION
FOR POLYCHLORINATED DIBENZO-P-DIOXINS AND DIBENZOFURANS
Vladimir S. Iorish
Glushko Thermocenter of Russian Academy of Sciences
"IVTAN" Association of RAS, HEDRC
Izhorskaya 13/19
Moscow, 127412, Russian Federation
ABSTRACT
Critical constants are important thermophysical parameters of substances in the field of chemical process design. They are used, for example, in extrapolating the vapor pressures from the normal boiling point to the critical temperature or in some other properties estimations [1].
Polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) are the most acutely toxic compounds in the environment. These pollutants are known to be by-products in chemical technology and also under various fuel and waste combustion. The availability of thermodynamic data for these compounds is of fundamental importance for understanding of the mechanism of their formation in order to assist in design of strategies to effectively control or eliminate their emission.
There are 75 isomers of PCDDs and 135 isomers of PCDFs. In absence of experimental data on critical constants some estimations should be made [2].
In this paper group contribution technique is used to estimate the critical constants for all isomers of PCDDs and PCDFs.
NOMENCLATURE
2378T4CDD 2,3,7,8 - tetrachlorodibenzo-p-dioxin
aL, bL coefficients in equation CpLo(T) = aL+ bLT/104 for liquid phase
aS, bS coefficients in equation CpSo(T) = aS+ bST/104 for solid phase
Cpo isobaric heat capacity
CpGo isobaric heat capacity of gas phase
CpL isobaric heat capacity of liquid phase
CpS isobaric heat capacity of solid phase
DD dibenzo-p-dioxin
DF dibenzofuran
DmHo=![]() mHo(Tm) enthalpy of fusion at Tm
mHo(Tm) enthalpy of fusion at Tm
DsHo=![]() sHo(298.15) enthalpy of sublimation
sHo(298.15) enthalpy of sublimation
DvHo(Tb) enthalpy of vaporization at boiling point
DvSo(Tb) entropy of vaporization at boiling point
M molecular weight
n number of atoms in a molecule
Np pressure index of a molecule
np pressure index of group
Nt temperature index of a molecule
nt temperature index of group
Nv volume index of a molecule
nv volume index of group
Pc critical pressure
PCDDs polychlorinated dibenzo-p-dioxins
PCDFs polychlorinated dibenzofurans
R gas constant
So= So(298.15) standard entropy
Tb normal boiling temperature
Tc critical temperature
Tm melting temperature
Tr = T/Tc
Vc critical volume
w acentric factor
x chlorine substitution
INTRODUCTION
The procedures for the estimation of critical constants of organic compounds have been recently reviewed by Somayajulu [3] and modified approach to the calculation has been proposed.
The equations for the estimation of the critical properties developed in [3] and used in this study are the following.
Tc = Tb + Tb / (at + bt Nt ) (K) (1)
Pc = M / (ap + bp Np )2 (bar) (2)
Vc = (av + bv Nv ) (cm3 mol-1) (3)
where Nt = S nt , Np = S np, Nv = S nv, at = 1.242, bt = 0.138, ap = 0.339, bp = .226 , av = 40.0, bv = 55.0.
Chirico et al. [4] demonstrated excellent agreement between the experimental values and those calculated by equations (1) - (3) for dibenzofuran that is shown in Table 1.
Table 1. Critical constants of dibenzofuran
|
Tc/K |
Pc/bar |
Vc/cm3 mol-1 |
|
|
Exp. data [4] |
824 |
36.36 |
495 |
|
Calculation |
826 |
36.5 |
499 |
The calculation requires molecular weight, normal boiling point and group indices. I used group indices listed by Somayajulu [3] and the additional increments for the macro group of dibenzofuran based on experimental data [4].
The normal boiling points are calculated from far extrapolation of vapor pressures measured over solid compounds [5]. The procedure requires thermodynamic functions estimation for gaseous and condensed phases [6]. I calculated also acentric factor w which is closely related to critical properties and defined as {-lg(P/Pc) - 1}, where P is the vapor pressure at T = 0.7Tc.
Method of calculation
Molecular indices for dibenzo-p-dioxin, dibenzofuran and their chlorinated derivatives are calculated from three group indices, listed in Table 2:
Na(PCDDs) = na1 + na2 + x na3, (4)
Na(PCDFs) = na1 + x na3, where a = p, t, v (5)
Table 2. Indices of groups
|
Group |
No |
nt |
np |
nv |
|
DF |
1 |
6.227 |
8.017 |
8.267 |
|
- O - (ring) |
2 |
0.800 |
0.710 |
0.363 |
|
- Cl (aromatic) |
3 |
0.642 |
1.400 |
0.801*) |
*)
The index was corrected for displacement of hydrogen atom by chlorine atom in aromatic ring.There are no experimental data on boiling points of DD, PCDDs and PCDFs. I calculated them from enthalpies of sublimation, experimental and estimated heats of fusion and thermodynamic functions of solid, liquid and gaseous substances obtained recently from combined consideration of all experimental and theoretical data about these compounds [6]. The procedures used for these estimations are briefly outlined below.
The values of the ideal gas entropy derived from calorimetric measurements are known for DF only[4]. The entropies and heat capacities of gaseous DD, PCDDs and PCDFs were estimated by group additivity approach and by statistical thermodynamics method [7,8,9]. The S o(T) and Cpo(T) values evaluated in these works differ from one another by 10 - 60 J.K-1.mol-1. Recent ab initio calculation of vibrational spectra of four tetrachlorodibenzo-p-dioxins [10] makes it possible to predict the values of entropy and heat capacity of PCDDs and PCDFs more reliably.
Structural parameters and vibrational frequencies needed for statistical thermodynamics calculation were estimated in this work using the similarity transference procedure. Based on X-ray diffraction and theoretical data for DD, DF and some of PCDDs and PCDFs, the structural parameters were evaluated to be the same for all PCDDs and PCDFs. Available vibrational assignments for DF, PCDDs as well as for chlorinated benzenes, anthracene, 1,4-dioxin, and furan were used in this work to develop the simplified force field approximation for PCDDs. The distinguishing feature of proposed method is that the force constants are calculated to best fit not only the vibrational frequencies, but also the experimental data on entropy or heat capacity if the later are known in the literature. Twenty-six force constants were obtained by a least-squares refinement to provide a satisfactory fit to known fundamentals of DF, PCDDs, PCDFs, chlorinated benzenes and to the experimental values of entropy of DF. Using estimated structural parameters and vibrational frequencies, the thermodynamic functions of all isomers of PCDDs and PCDFs were calculated in this work by rigid-rotor harmonic-oscillator approximation.
The only experimental information on thermodynamic functions of DD, PCDDs and PCDFs in condensed phase is indirectly contained in several vapor pressure measurements for solid [5] and overcooled liquid [11] compounds. To extract the data on thermodynamic functions from these experimental results some assumptions on heat capacity of solid and liquid phases should be made. Domalsky and Hearing[12] showed the possibility of satisfactory estimation of the heat capacity for solid organic compounds at 298.15K by group additivity method. In the absence of all required increments for the heat capacity of PCDDs and PCDFs estimation, "difference method" was used, which is fully consistent with the group additivity approach. Using experimental heat capacity values for several related compounds CpSo(DD, 298.15) = 215 ![]() 5 J.
K-1.
mol-1 was estimated. From this value and increments for chlorine substitutions developed by Domalsky and Hearing[12] heat capacities(in J.
K-1.
mol-1) for all solid PCDDs and PCDFs were estimated by equations:
5 J.
K-1.
mol-1 was estimated. From this value and increments for chlorine substitutions developed by Domalsky and Hearing[12] heat capacities(in J.
K-1.
mol-1) for all solid PCDDs and PCDFs were estimated by equations:
CpS(xCl-DD) = CpS(DD) + 13.42 x,
CpS(xCl-DF) = CpS(DF) + 13.42 x, (6)
where CpS(DF) = 199.01 J. K-1. mol-1 [4].
To extrapolate the heat capacity from room temperature up to the melting point the linear equation derived from experimental data for related compounds is used:
CpS(T)/R = CpS(298.15)/R + 1.51. n. (T – 298.15)/Tm (7)
Bondi-Rowlinson equation [1], the heat capacities for gases, critical temperatures and acentric factors were used for heat capacity estimation of liquid DD, PCDDs and PCDFs:
CpL /R= CpGo/R + 2.56 + 0.436. (1 – Tr)-1 + w. [2.91 +
4.28. (1 – Tr)1/3Tr-1 + 0.296. (1 – Tr)-1] (8)
So several iterations were needed to agree the data. The final heat capacities were approximated by linear dependence on temperature (see Table 4).
Vapor pressure data for several PCDDs and PCDFs [5] were treated by "Third Law" and "Second Law" techniques taking into account direct determination the enthalpy of sublimation for some of PCDDs[13,14]. As a result of these treatments standard entropy values and enthalpies of sublimation were estimated for the considered solid PCDDs and PCDFs. Rordorf's procedure [5] for enthalpies of fusion estimation is used for the cases where the values were not known from experiment.
The thermodynamic functions were correlated with chlorine substitution and the following equations allow estimate the same data for all other PCDDs:
So(298.15) = 214.8 + 23.02 x J. K-1. mol-1 ,
DsHo(298.15) = 91.6 + 7.93 x kJ. mol-1 ,
DmHo = 15.7 + 5.37 x kJ. mol-1 ,
aL = 149.9 +31.42 x J. K-1. mol-1 ,
bL = 3971 - 292.0 x J. mol-1. 10000,
and PCDFs:
So(298.15) = 196.2 + 21.625 x J. K-1. mol-1,
DsHo(298.15) = 88.5 + 8.14 x kJ. mol-1,
DmHo = 21.4 + 3.87 x kJ. mol-1,
aL = 124.0 + 32.82 x J. K-1. mol-1
bL = 4045 - 320.74 x J. mol-1. 10000. (9)
Selected data for DD, 2378-T4CDD and 2378-T4CDF are presented in Tables 3 and 4 as an example.
Table 3. Thermodynamic functions for gas phase (in J. K-1. mol-1)
|
Substance |
So(298) |
Cpo(T) |
||||||
|
298.15 |
400 |
600 |
800 |
1000 |
||||
|
DD |
396.646 |
180.242 |
239.191 |
325.449 |
380.428 |
417.09 |
||
|
2378- T4CDD |
510.477 |
241.176 |
297.504 |
374.867 |
421.241 |
450.439 |
||
|
2378- T4CDF |
489.659 |
224.302 |
278.924 |
354.064 |
399.081 |
427.450 |
||
Table 4. Thermodynamic functions for condensed phase (in J.
K-1.
mol-1 : So,aS, aL and in kJ.
mol-1: ![]() sHo and
sHo and ![]() mHo)
mHo)
|
Substance |
DsHo |
So |
Tm/K |
DmHo |
aS |
bS |
aL |
bL |
|
DD |
90.8* |
211.5 |
392.5* |
21.9* |
5.16 |
7038 |
143.4 |
4127 |
|
2378-T4CDD |
127.7* |
312.0 |
578.2* |
38.9* |
126.56 |
4777 |
274.5 |
2830 |
|
2378-T4CDF |
121.1 |
282.7 |
500.7* |
36.9 |
93.17 |
5266 |
255.3 |
2762 |
*)
This value is based on experimental dataUsing the data described above the normal boiling temperatures were calculated from the equation:
DvGo(Tb)= DvSo(Tb) - DvHo(Tb)/Tb = 0 (10)
Gas imperfection correction is not considerable (2-3K) and not taken into account.
Results and discussion
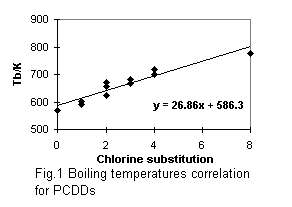
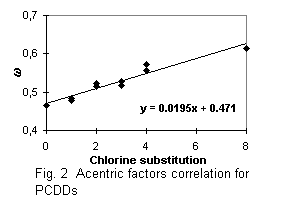
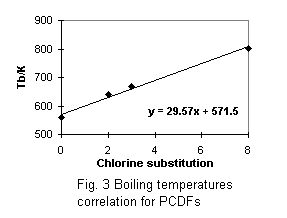
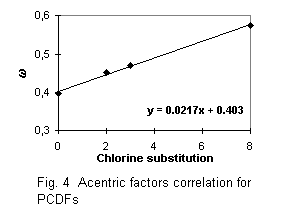
Calculated critical constants are presented with obtained normal boiling temperatures in Tables 5 and 6. The first parts of these tables refer to PCDDs and PCDFs for which experimental data on vapor pressures were used for the boiling points estimation. The correlation equations for boiling temperatures and acentric factors (see Fig. 1 - 4 ) were used in the critical temperatures and acentric factors calculation for other chlorinated substances, presented in the second part of Tables 5 and 6. I compare the critical constants estimated in this paper with data predicted in [2] (see Table 5). Considerable difference in estimated Tc values is explained by different group indices and boiling temperatures.
Uncertainties in the predicted critical constants one can estimate as 10 - 30 K for Tc, 1 - 3 bar for Pc and 10 - 30 cm3 mol-1 for Vc in dependence on chlorine substitution.
Table 5.
|
Substance |
Tc K |
Pc bar |
Vc cm3 mol-1 |
w |
Tb K |
|
DD |
827 |
34.52 |
515 |
0.466 |
569 |
|
1-CDD |
846 |
31.66 |
559 |
0.484 |
590 |
|
2-CDD |
861 |
31.66 |
559 |
0.478 |
600 |
|
23-DCDD |
887 |
29.20 |
603 |
0.515 |
625 |
|
27-DCDD |
932 |
29.20 |
603 |
0.522 |
657 |
|
28-DCDD |
950 |
29.20 |
603 |
0.514 |
670 |
|
137-T3CDD |
959 |
27.05 |
647 |
0.517 |
683 |
|
124-T3CDD |
936 |
27.05 |
647 |
0.529 |
667 |
|
1234-T4CDD |
973 |
25.17 |
691 |
0.555 |
700 |
|
2378-T4CDD |
997 |
25.17 |
691 |
0.573 |
717 |
|
2378-T4CDD T4CDD[2] |
934* |
23.42* |
763* |
694* |
|
|
OCDD |
1043 |
19.61 |
867 |
0.614 |
777 |
|
Other PCDDs: |
|||||
|
DCDDs |
908 |
29.20 |
603 |
0.510 |
640 |
|
T3CDDs |
936 |
27.05 |
647 |
0.530 |
667 |
|
T4CDDs |
964 |
25.17 |
691 |
0.549 |
694 |
|
PCDDs |
992 |
23.51 |
735 |
0.569 |
721 |
|
H6CDDs |
1020 |
22.06 |
779 |
0.588 |
747 |
|
H7CDDs |
1048 |
20.76 |
823 |
0.608 |
774 |
*)
The value calculated in [2]Table 6.
|
Substance |
Tc K |
Pc bar |
Vc cm3 mol-1 |
w |
Tb K |
|
DF [3] |
824 |
36.4 |
495 |
0.397 |
558.3 |
|
36-DCDF |
923 |
30.6 |
583 |
0.452 |
642 |
|
248-T3CDF |
951 |
28.3 |
627 |
0.472 |
669 |
|
OCDF |
1088 |
20.2 |
847 |
0.574 |
802 |
|
Other PCDFs: |
|||||
|
MCDFs |
876 |
33.29 |
539 |
0.425 |
601 |
|
DCDFs |
907 |
30.60 |
583 |
0.446 |
631 |
|
T3CDFs |
939 |
28.25 |
627 |
0.468 |
660 |
|
T4CDFs |
971 |
26.21 |
671 |
0.490 |
690 |
|
PCDFs |
1002 |
24.43 |
715 |
0.512 |
719 |
|
H6CDFs |
1033 |
22.86 |
759 |
0.533 |
749 |
|
H7CDFs |
1065 |
21.48 |
803 |
0.555 |
778 |
Acknowledgments
This work was supported by the Russian Fund for Basic Research Grants No. 96-02-16223 and No. 96-07-89026. The author would like to thank Dr. V.Yu.Zitserman for fruitful discussions.
References
[1] Reid, R.C., Prausnitz, J.M. and Sherwood, T.K., 1977, The Properties of Gases and Liquids, Chapters 2 and 5. Third edition. McGraw-Hill Book Company, New York.
[2] Schroy, J.M., Hilman, F.D. and Cheng, S.C. , 1985, Physical/Chemical properties of 2,3,7,8-TCDD, Chemosphere, Vol. 14, pp.873-876.
[3] Somayajulu, G.R., 1989, Estimation Procedures for Critical Constants, J.Chem.Eng.Data., Vol. 34, pp. 106-120.
[4] Chirico, R.D., Gammon, B.E., Knipmeyer, S.E., Nguyen, A., Strube, M.M., Tsonoloulos, C. and Steele, W.V., 1990, The thermodynamic properties of dibenzofuran, J. Chem. Thermodynamics, Vol. 22, pp. 1075-1096.
[5] Rordorf, B.F.,1989, Prediction of vapor pressures, boiling points and enthalpies of fusion for twenty-nine halogenated dibenzo-p-dioxins and fifty-five dibenzofurans by vapor pressure correlation method, Chemosphere, Vol. 18, pp.783-788.
[6] Iorish, V.S., Dorofeeva, O.V. and Moiseeva, N.F., 1998, Thermodynamic properties of dibenzo-p-dioxin, dibenzofuran and their polychlorinated derivatives in the gaseous and condensed phases, Chemosphere (in press).
[7] Shaub, W. M., 1982, Estimated thermodynamic functions for some chlorinated benzenes, phenols and dioxins, Thermochim. Acta , Vol. 58, pp. 11-14.
[8] Thompson, D., 1995, Enthalpies of formation and entropies of chlorinated dibenzo-p-dioxins and dibenzofurans; selected data for computer-based studies Thermochim. Acta, Vol. 261, pp. 7-20.
[9] Dorofeeva, O.V. and Gurvich, L.V., 1996, Thermodynamic properties of polychlorinated dibenzo-p-dioxins and furans in the gas phase, Zh. Fiz. Khim. Vol. 70, pp. 7-12.
[10] Rauhut, G., Pulay, R., 1995, Identification of isomers from calculated vibrational spectra. A density functional study of tetrachlorinated dibenzodioxins. J.Am.Chem Soc., Vol. 117, pp. 4167-4172.
[11] Eltzer, B.D., and Hits, R.A., 1988, Vapor Pressures of Chlorinated Dioxins and Dibenzofurans, Environ.Sci.Technol, Vol. 22, pp. 1362-1364.
[12] Domalski, E. S., and Hearing, E. D., 1993, Estimation of the Thermodynamic Properties of C-H-N-O-S-Halogen Compounds at 298.15 K, J.Phys. Chem. Ref. Data, Vol. 22, pp. 805-1159.
[13] Papina, T. S., Kolesov, V.P., Vorobieva, V.P., and Golovkov, V.F., 1996, The standard molar enthalpy of formation of 2-chlorodibenzo-p-dioxin, J. Chem. Thermodynamics, Vol. 28, pp.307-311.
[14] Luk'yanova, V.A., Kolesov, V.P., Avramenko, N.V., Vorobieva, V.P., and Golovkov,V.F., 1997, Standad enthalpy of formation of dibenzo-p-dioxin, Russian J.Phys.Chem, Vol. 71, pp.338-340.