The velocity of chemical reactions. Additional material.
Nuance 1:
This expression
v = Dc/Dt
allows to determine only average velocity of reaction for a given time interval.
But scientists, as a rule, are interested in the velocity at a given moment of time,
i.e. so-called moment velocity of reaction. It is defined as a derivative of function
c(t):
v = dc/dt
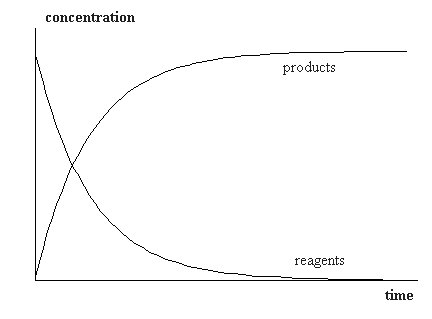
If we determine the velocity of reaction by one of the reagents then derivative
c(t) has a negative value since concentration of reagents decrease. But this is impossible,
as it does not comply with the physical meaning. Therefore the formula is modified
to have physical sense
v = -dc/dt
Nuance 2:
Let's determine the velocity of the same reaction
H2 + I2 = 2HI
Not by decrease of concentrations of reagents, but by increase of concentration of products
v(HI) = dc(HI)/dt
v(H2) is equal to v(I2), but unequal to v(HI), as if the concentrations
of reagents decrease in 3 times, the concentration of HI would increase in 2*3=6 times!. To make
speaking about the overall velocity of reaction possible, the rate of the reaction should be divided
by the coefficient:
v(HI) = dc(HI)/2dt
In general case, for the reaction
aA + bB = eE + fF
the velocity is
v = -dc(A)/adt = -dc(B)/bdt = dc(E)/edt = dc(F)/fdt
Supplementary
| 



